Who We Are
MEZOO, a leader in ambulatory Remote
Patient Monitoring (aRPM).
Founded in 2018, Mezoo is a specialized digital healthcare company leading the field of
wearable-based ambulatory Remote Patient Monitoring (aRPM).
MEZOO, short for Medical Equipment ZOO, embodies a vision of becoming a core player in the digital healthcare ecosystem through a wide range of IoMT solutions. Guided by the mission “technology that protects life, innovation that transforms living,” Mezoo delivers safe and convenient healthcare services for patients, efficient clinical environments for medical professionals, and improved operational efficiency for healthcare institutions.

Business Areas & Core Technologies
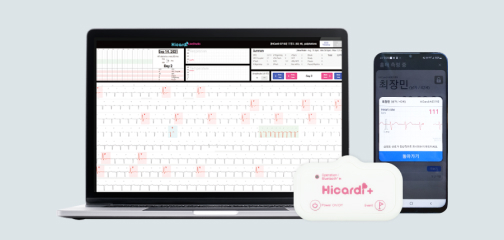
Mezoo provides ambulatory Remote Patient Monitoring (aRPM) solutions that connect care inside
and outside the hospital, powered by biomedical telemetry technology.
-
 01
01Wearable Patch
-
 02
02Gateway
-
 03
03Monitoring Web Viewer
Key Achievements
-
01Partnerships
Dong-A ST (strategic investor and commercial partner)
-
02Regulatory Approvals
17 total approvals including MFDS, CE MDD, FDA 510(k), GMP, PMDA, ANVISA.
History
Every step of yesterday shapes today and opens tomorrow.
Technology Development





- 01
-
Company founded by four PhD researchers in Biomedical Engineering from Yonsei University.
Initiated development of wearable healthcare technology.
- 11
-
Secured core patents for wearable monitoring systems.
Commenced full-scale R&D in biomedical hardware technology.
- 10
-
Completed healthcare platform development for smart wearable devices.
Established advanced biomedical hardware capabilities.
- 09
-
Secured patents for ECG electrodes and electrode modules.
- 12
-
Completed development of high-end diagnostic ECG algorithms.
- 03
-
Established corporate research lab.
Advanced software technology capabilities.

- 04
-
Transitioned to corporation and initiated full commercialization.
Focused development on aRPM technology.
- 02
-
Official launch of HiCardi.
- 12
-
Awarded by the Ministry of SMEs and Startups’ “Big Star Solver Platform.”
- 02
-
Designated as an Innovative Product by the Public Procurement Service.
- 05
-
Obtained CE certification and established foundation for global expansion.
- 12
-
Completed large-scale clinical validation (2,000 cases).
Verified commercialization of SmartView and LiveStudio platforms.

- 02
-
Official domestic launch of HiCardi+.
Obtained MFDS approval for combined patient monitor + Holter functionality.
- 07
-
Formed strategic partnership with Dong-A ST.
Began exclusive domestic distribution and joint marketing.
- 08
-
Released HiCardi+ H100 with enhanced Holter functionality.
Expanded adoption across major tertiary hospitals.
Global expansion and next-generation technology development (2024–present).

- 09
-
Obtained U.S. FDA 510(k) clearance.
Completed certifications from Japan PMDA and Saudi Arabia SFDA.
- 03
-
Launched new HiCardi M300.
Advancing next-generation technologies and global market expansion.
