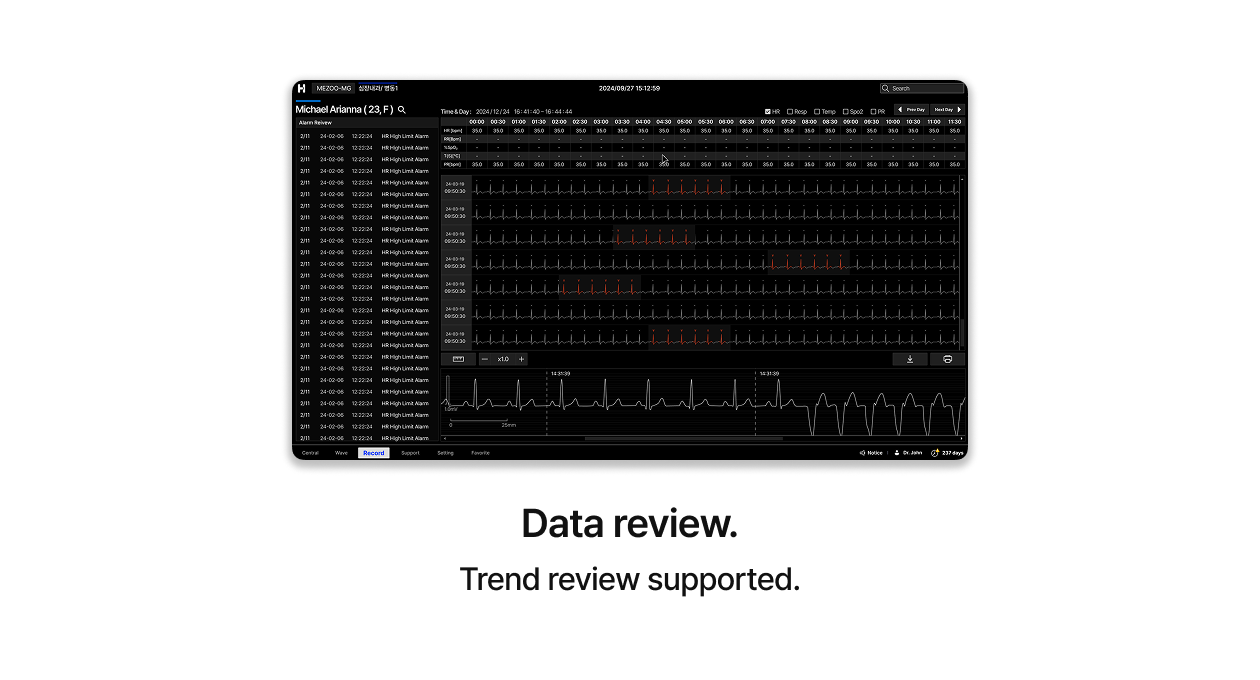
LiveStudio
One person, unified monitoring.
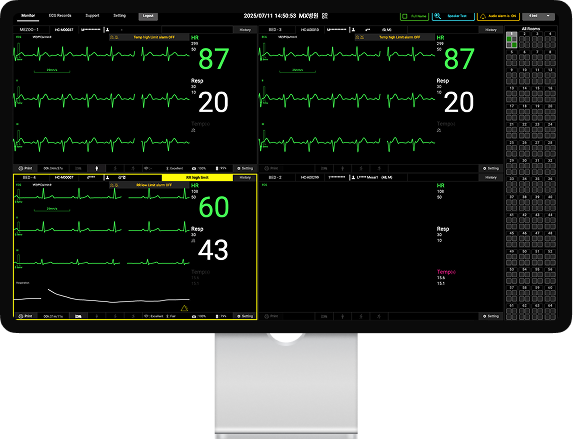
Key Features
Regulatory Information
*This product is registered as a component of LiveStudio. This product is a medical device. Please read the “Precautions for Use” and “Instructions for Use” carefully.
Advertising review approval number: 52025-I10-42-4782 (Valid until 28.11.20).
Advertising review approval number: 52025-I10-42-4782 (Valid until 28.11.20).
- Model Name
-
LiveStudio
- Product Type:
- Patient Monitoring Software
- Intended Use:
- LiveStudio collects signals generated from multiple biosignal measuring devices—such as patient monitors, Holter ECGs, and pulse oximeters—then displays, stores, analyzes, and retrieves them, enabling centralized monitoring of all acquired biosignals.
- Certification number
- 23-4617
- Manufacturer
- Mezoo Co., Ltd.
- Method of use and
precautions for use - Refer to the instructions for use (IFU)
- Customer Support Center
- (+82) 1660-1056